Introduction: The optimal induction regimen for an allogeneic hematopoietic cell transplant (allo-HCT) for a young adult patient diagnosed with acute myeloid leukemia (AML) remains to be defined. Among reduced intensity regimens (RIC), retrospective registry data from both CIBMTR and EBMT would suggest that a Fludarabine / Melphalan (Flu/Mel) regimen was superior to a Fludarabine / Busulfan x 2 (Flu/Bu2) regimen. The need for a myeloablative conditioning regimen over a RIC has been evaluated in at least six randomized clinical trials (RCT), and the results have yet to be uniform. Published meta-analysis suggests equipoise. The studies are further limited by including a heterogenous group of conditioning regimens, especially in the reduced intensity arm; they all come from developed countries, a mix of AML and MDS in most studies, and variation in age groups enrolled in them. In India and developing countries, there remain significant challenges with multi-drug resistant infections, financial constraints, and challenges with intensive care support. We hypothesize that in this setting, a reduced toxicity regimen, such as a combination of Fludarabine with Melphalan (140 mg/m 2) (Flu/Mel), may be preferable to a myeloablative regimen.
Methods: A single-center retrospective analysis was undertaken to study the impact of a reduced toxicity Flu/Mel regimen versus a myeloablative Fludarabine combined with Busulfan (130 mg/m 2/day x 4 days) regimen (Flu/Bu4). All consecutive patients in all age groups with a diagnosis of AML who underwent an allo-HCT between Jan 2005 and Dec 2021 were included in this analysis. The conditioning regimen used was as per physician discretion with a broad guideline within the department to use a myeloablative Flu/Bu4 regimen in a young fit patient with high-risk features such as high-risk ELN at diagnosis, CR2, and beyond, or requiring more than one cycle of induction chemotherapy to achieve a complete remission (CR).
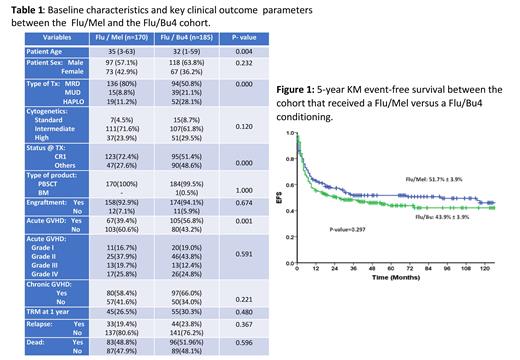
Results: 439 patients underwent an allo-HCT for a diagnosis of AML at our center in the given time. The median age was 33 years (range: 1 - 63), and there were 267 (60.8%) males. The transplants were done in CR1 in 236 (54%), CR2 in 90 (20.5%), CR3 in 13 (3%), and in relapsed/refractory or with active disease in 100 (22.8%). The majority were matched sibling/related donors (MRD) in 284 (64.7%), followed by matched unrelated (MUD) in 75 (17.1%) and haploidentical in 80 (18.2%). The conditioning regimen used was Flu/Mel in 170 (38.7%), Flu/Bu4 in 185 (42.1%), and a mix of other regimens in 84 (19.1%). At an actuarial median follow-up of 4 years, the overall survival (OS) and event-free survival (EFS) of the entire cohort were 40.8% ± 3.0% and 38.6% ± 3.0%, respectively. The EFS of cases in CR1, CR≥2, and in relapsed/refractory/active disease were 47.7% ± 3.8%, 45.0% ± 5.5%, and 12.3% ± 3.6%, respectively. The baseline characteristics and clinical outcome of cases that received a Flu/Mel condition versus Flu/Bu4 conditioning are summarized in Table 1. In summary, baseline characteristics were comparable between the two groups, except that significantly more cases underwent a haploidentical transplant in the Flu/Bu4 group. In contrast, in the Flu/Mel group, there were significantly more cases in CR1, and the median age was also significantly older. The 5-year KM EFS of the Flu/Mel and Flu/Bu4 cohort was 51.7±3.9% and 43.9±3.9% (P-value = 0.297), respectively (Figure 1). On univariate analysis, only CR1 status before transplant and the presence of chronic GVHD impacted EFS positively, and on multivariate analysis, only CR1 status affected EFS.
Conclusion: In conclusion, in one of the most extensive retrospective studies comparing a uniform reduced toxicity conditioning regimen with Flu/Mel versus a standard myeloablative Flu/Bu4 conditioning regimen, we find no significant advantage of Flu/Bu4 over a Flu/Mel conditioning regimen.
Disclosures
Srivastava:Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Octapharma: Speakers Bureau; Biomarin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Spark: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Abraham:Pfizer: Research Funding; Sanofi: Research Funding; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal